// Project
Project INSPIRED
Duration of the project
48 months
Financing contract
92 of 09.09.2016
Partners
OncoGen-Pius Brânzeu Emergency County Clinical Hospital, Timișoara
Project details
PROJECT COMPLETION ANNOUNCEMENT
Project summary
Ambrosia artemisiifolia, the common ambrosia, also known as the meadow flower or fallow grass, is widespread in Romania. The pollen of this plant is known for its potential to cause allergic reactions in late summer and autumn and is a major health problem in Romania and neighboring countries. Vaccine-based diagnosis and treatment of ambrosia allergy may be ineffective due to the quality of the allergen extracts. The use of recombinant allergens can solve existing problems in diagnosis and treatment.
Objectives
The main objective of the INSPIRED project (Innovative strategies for the prevention, diagnosis and therapy of respiratory diseases induced by ambrosia pollen) is to develop a new diagnostic kit based on recombinant allergens that would enable effective personalized therapies for ragweed pollen allergy patients.
A secondary objective of the INSPIRED project is the evaluation of commercially available ambrosia pollen allergen vaccines. The results of this study, in combination with the diagnosis of ambrosia pollen allergy, will allow clinicians to predict the outcomes of allergen immunotherapy in patients.
Results
A panel of 9 ambrosia pollen allergens was produced in recombinant form (Fig. 1). Analysis of patient sera has shown that many patients are allergic to multiple allergens rather than just one, which means that each recombinant allergen needs to be evaluated (Fig. 2).
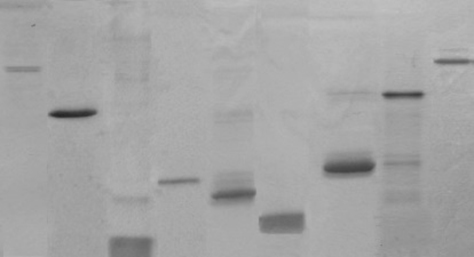
Figure 1: Recombinant allergens produced in the INSPIRED project. Different band sizes indicate different molecular weights of the allergenic proteins.
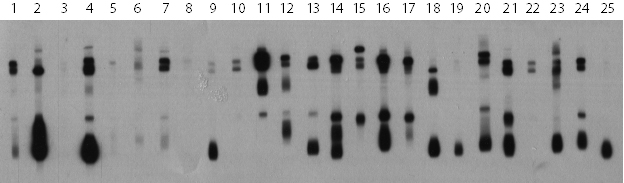
Figure 2: Patients allergic to different allergenic components of ragweed pollen as indicated by bands of different sizes and intensities
ELISA analysis revealed different IgE reactivity to individual allergens (Table 1). Some of this IgE reactivity may be due to cross-reactivity to allergens from other sources, such as Artemisia (pelinarium). Quantitative IgE evaluation was also performed in the ImmunoCAP system (Fig. 3), to confirm IgE reactivity profiles.


Figure 3: The ImmunoCAP Phadia 250 System, found at the OncoGen Center, is used to measure IgE
With the recombinant allergens produced at the OncoGen Center, it is possible to determine a precise allergen reactivity profile of each allergic patient. In collaboration with the Medical University of Vienna, the recombinant allergens will be included in a chip, which will allow a fast and accurate personalized diagnosis (Fig. 4).
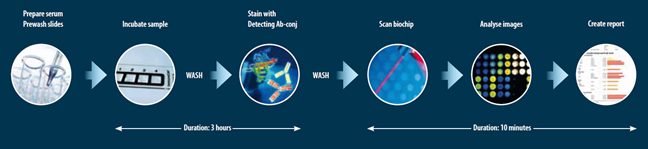
Figure 4: Allergen chip technology

International Asthma Day / World Asthma Day Prof. Carmen Panaitescu & Assoc Prof. Roxana Bumbacea, RFI. Interview conducted on: 05.05.2021

COVID-19 and anaphylactic reaction / COVID-19 and Anaphylactic Reaction, Prof. Carmen Panaitescu, RFI.Interview conducted on: 14.12.2020