
// Project
In-vitro differentiation of mesenchymal stem cells
Period of deployment
2009 – 2011
Project no.
ID 1748/2008
Coordinator
"Victor Babeș" University of Medicine and Pharmacy in Timișoara
Funding:
1,000,000 RON
Full name
Studies on the in vitro differentiation of mesenchymal stem cells towards the adipocyte lineage and the validation of molecular factors involved in adipogenesis
Objectives
General objectives: (1) determining the optimal methods and conditions by which adult mesenchymal stem cells can be induced to differentiate towards the adipocyte lineage; (2) defining and validating the molecular control mechanisms involved in MSC differentiation towards adipocytes.
Specific objectives: (1) optimizing the methods of obtaining, isolating and cultivating adult human mesenchymal stem cells from hematogenous bone marrow in order to induce differentiation towards the adipocyte lineage; (2) the highlighting of external modulators of adipocyte differentiation, from induction to terminal differentiation; (3) verification of adipocyte differentiation by immunohistochemical and molecular biology methods; (4) evaluation and correlation of the results obtained from the perspectives of informational integration in the context of the development of obesity; (5) investigating the factors involved in the transcriptional control of adipocyte genes during differentiation; (6) verifying the effectiveness of the inhibition of the MSC differentiation process towards the adipocyte line; (7) validation and optimization of transfection protocols used to inhibit adipogenesis; (8) developing a theoretical concept regarding the complex mechanisms involved in the appearance of obesity
Project summary:
From the perspective of the increasing prevalence of obesity worldwide, even among children, the present project proposed a systematized approach to the molecular factors involved in the differentiation of mesenchymal stem cells towards the adipocyte lineage. After the harvesting of the human hematogenous bone marrow and the isolation of the MSCs, the development of optimal differentiation protocols was done, using inducing and maintaining agents of adipogenesis. Adipocyte differentiation was verified by specific immunohistochemical and molecular biology methods, subsequently establishing the genes involved in the adipogenesis process by the microarray technique. The identification of molecular markers involved in adipogenesis, especially in the early stages of differentiation of human MSCs to pre-adipocytes, opens important practical perspectives for further therapeutic approaches in the treatment of obesity. Once these markers are identified and the gene expression involved in adipogenesis established, it will be possible to identify modern methods of inhibiting the process of adipose tissue formation, targeting at the molecular level. So far, the genes that regulate adipogenesis have not been clearly identified, especially regarding the early phases of this process. In this regard, we used a modern transfection technique, which uses microRNA (miRNA). We identified by microarray techniques the genes that encode the processes of adipogenesis in different stages. After establishing the mRNA of interest, it was interfered with miRNA through the process of gene-silencing, to identify the key genes used in subsequent steps. Finally, we generated a genetic map and developed a theoretical concept regarding the complex mechanisms involved in the appearance of obesity.
Results:
From the point of view of the significant results generated by this project, we consider relevant the realization of the conceptual model for the identification and analysis of the genes involved in adipogenesis, both in the pre-adipogenesis stage and for mature adipocytes. Through the microarray technique, the genes that intervene in the regulation of the adipogenesis process were identified, which were clustered, showing particular interest in the genes involved in lipid metabolism. From the multitude of up-and down-regulated genes, the genes of interest were selected, which were inhibited by using specific siRNA sequences – PPARγ, ALOXB15, c-myc. However, the data obtained by chemical methods, such as GC-MS, showed that adipocytes differentiated in vitro from mesenchymal stem cells do not have the same composition of free fatty acids, which makes the in vitro differentiation model lead to the appearance of immature cells, with altered function. Although the interference phenomenon (siRNA) leads to the inhibition of MSC differentiation towards the adipocyte line, especially if it is carried out at the level of pre-adipocytes, the ability of MSC to differentiate into functional cells is questioned. The final result of these investigations was the establishment of the genetic map for adipocytes differentiated from human mesenchymal stem cells.
- Conceptual model for the identification of different groups of genes involved in adipogenesis
- Gene identification protocol through the microarray technique
- miRNA extraction protocol
- Model transfection concept/protocols
- Gene map of the main adipocyte genes
Participation in scientific events:
- Bojin F, Cristea M, Ordodi V, Anghel S, Tanasie G, Panaitescu C, Paunescu V. Differences between stromal stem cells from various tissue sources – morphological and functional studies. Romanian Journal of Biochemistry, 2011; 48(suppl.): 30 – The Annual International Conference of the Romanian Society of Biochemistry and Molecular Biology, The Conference on "Cellular and Molecular Biotechnologies on Medical Applications", September 28-30, Craiova, Romania.
- Ordodi V, Bojin F, Gruia A, Boleman A, Gavriliuc O, Cristea M, Anghel S, Tanasie G, Panaitescu C, Paunescu V. Mesenchymal stem cells as model of in vitro adipogenesis. Physiology-Physiology, 2011; suppl: 55 – 41St Annual Immunology Conference, September 22-24, Timisoara, Romania.
- Bojin F, Gavriliuc O, Tatu CA, Ordodi V, Cristea M, Gruia A, Anghel S, Boleman A, Crisnic D, Tatu C, Tanasie G, Panaitescu C, Paunescu V. Genetic changes during adipocytes ontogenesis. VIth Academician Nicolae Cajal Symposium, Bucharest, March 30-April 01 2011, Abstract book pg. 19.
- Bojin F, Gavriliuc O, Tatu CA, Cristea M, Anghel S, Crisnic D, Nistor D, Tatu C, Tanasie G, Panaitescu C, Paunescu V. In vitro adipogenic differentiation pathway. The XXVth National Conference of Romanian Society of Physiological Sciences, 26-28 May 2011, Tg. Mures, Romania
- Paunescu V, Bojin F, Gavriliuc O, Tatu CA, Ordodi V, Rosca A, Cristea M, Gruia A, Anghel S, Nistor D, Crisnic D, Tatu C, Tanasie G, Panaitescu C. Multipotent stromal cells of different origins: insights on in vitro characteristics. Physiology-Physiology, 2011; suppl: 33 – 41St Annual Immunology Conference, September 22-24, Timisoara, Romania.
- Bojin MF, Gavriliuc O, Tatu CA, Bunu C, Paunescu V. Tumor-associated fibroblasts recapitulate in vitro adipogenic differentiation pathway. Obesity Reviews 2010; 11th International Congress on Obesity, 11-15 July, Stockholm, Sweden – Abstract Book, pp. 92-93.
- Bojin F, Tatu CA, Gavriliuc O, Boleman A, Gruia A, Cristea M, Anghel S, Nistor D, Tatu C, Tanasie G, Bunu C, Paunescu V. Adipocytes-induced changes in tumor microenvironment and cancer cells behavior. Immunology and Clinical Allergology Joint Meeting, 28 April-1 May 2010, Sibiu, Romania – Abstracts, pg. 30, ISBN 978-973-88744-3-5.
- Bojin FM, Tatu CA, Gavriliuc O, Ciuculescu F, Cristea M, Rosca A, Anghel S, Gruia A, Crisnic D, Tanasie G, Tatu C, Nistor D, Bunu C, Paunescu V. In vitro characterization of tumor-associated fibroblasts as supportive network for cancer development. International Immunology 2010, 22 (suppl.); 167 – 14th International Congress of Immunology, Kobe, Japan.
- Bojin F, Gavriliuc O, Tatu C, Tanasie G, Tatu C, Nistor D, Crisnic D, Boleman A, Bunu C, Paunescu V. Adipogenic differentiation pathway – gene expression profile. Physiology – Physiology, 2010; supplement, Vol. Abstracts, National Conference of the Romanian Society of Physiological Sciences, June 2-5, 2010, Oradea, Romania, pg. 33.
Monograph:
Florina Bojin. Adipocytes – in vitrodifferentiation models. Eurostampa Publishing House, Timisoara 2010, ISBN 978-606-569-139-1
Full articles:
- Bojin FM, Gavriliuc OI, Cristea MI, Tanasie G, Tatu CS, Panaitescu C, Paunescu V. Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med. 2011; 15(10):2269-2272.
- Bojin FM, Gruita AT, Cristea MI, Ordodi VL, Paunescu V, Mic FA. Adipocytes Differentiated In Vitro from Rat Mesenchymal Stem Cells Lack Essential Free Fatty Acids Compared to Adult Adipocytes. Stem Cells and Development. 2011, doi:10.1089/scd.2011.0491
- Oana I. Gavriliuc, Florina M. Bojin, Adriana Rosca, Calin A. Tatu, Virgil Paunescu. Silencing of the Her2 Gene by RNA Interference Inhibits Proliferation of Breast Cancer Cell Line SK-BR3. Physiology-Physiology, 2011, vol. 21, no. 3 (71), pp. 4-9. ISSN 1223-2076
- Florina Bojin, Oana Gavriliuc, Valentin Ordodi, Mirabela Cristea, Simona Anghel, Carmen Tatu, Gabriela Tanasie, Carmen Panaitescu, Virgil Paunescu. Controversies Related To Cell Cultures Obtained From Various Tissue Samples. Physiology-Physiology, 2011, vol. 21, no. 4 (72). ISSN 1223-2076
- Bojin F, Tatu CA, Gavriliuc O, Gruia A, Tanasie G, Tatu C, Cristea M, Ciuculescu F, Tocut A, Crisnic D, Carabineanu A, Nistor D, Bunu C, Paunescu V. Stromal cells – tumor microenvironment interactions – Part I. Physiology-Physiology, 2010, vol. 20, no. 1 (65), pp. 37-41. ISSN 1223-2076
- Bojin F, Tatu CA, Gavriliuc O, Gruia A, Tanasie G, Tatu C, Anghel S, Cristea M, Ciuculescu F, Tocut A, Crisnic D, Carabineanu A, Nistor D, Bunu C, Paunescu V. Stromal cells – tumor microenvironment interactions – Part II. Physiology-Physiology, 2010, vol. 20, no. 2 (66), pp. 31-36. ISSN 1223-2076
- Bojin F, Ordodi V, Anghel S, Gruia A, Gavriliuc O, Georgescu R, Vintila R, Tatu C, Bunu C, Tanasie G, Paunescu V. Mesenchymal stem cells admix with biological scaffolds heal bone defects in rat model. Romanian Biotechnological Letters, ISSN 1224-5984.
Prizes:
- "Young Researchers - with experience" on the occasion of The XXVth National Conference of Romanian Society of Physiological Sciences, 26-28 May 2011, Tg. Mures, Romania, the work entitled "In vitro adipogenic differentiation pathway"
- "Grigore Ghyka" - Clinical Immunology prize for "Adipocytes-induced changes in tumor microenvironment and cancer cells behavior" poster presentation in Joint Meeting Immunology and Clinical Allergology, 28 April-1 May 2010, Sibiu, Romania
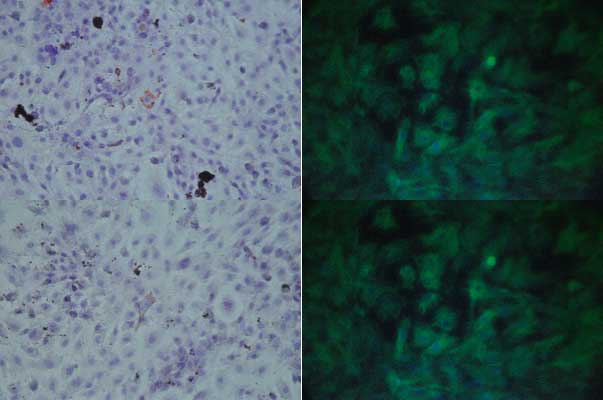
Fig. 1. Undifferentiated MSCs (top) and pre-adipocytes (bottom). Staining Oil Red O (left) and IF Vimentina (right) Ob. 40x
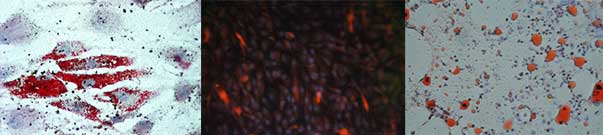
Fig. 2. Mature adipocytes differentiated from MSCs (top) Oil Red O staining (left) and IF for FABP4 (right). Adult adipocytes from adipose tissue - cytospin, Oil Red O staining (bottom). Ob. 40x
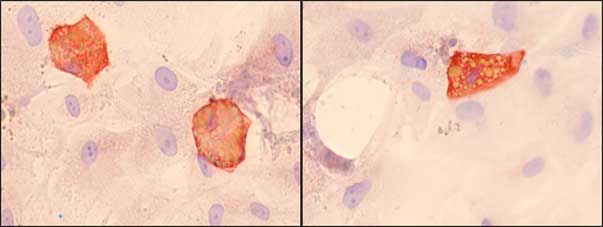
Fig. 3. Immunocytochemistry for adipocytes derived from MSC (left) and TAF (right) (FABP4) after 21 days of culture. Lipid vacuoles (yellow) intracytoplasmic (orange), counterstaining with Hematoxylin solution (Ob. 100x). A higher rate of differentiation is observed in the case of MSC compared to TAF
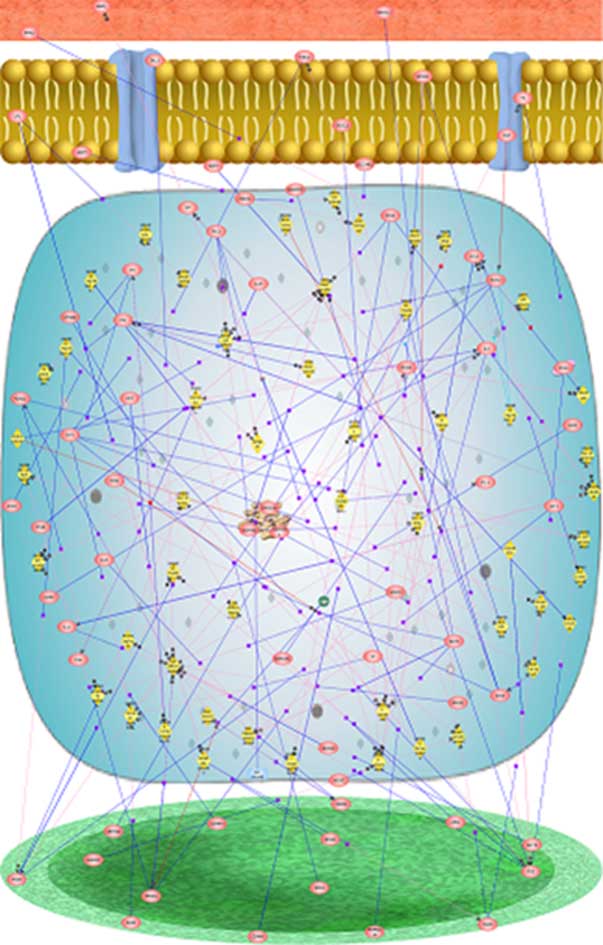
Fig. 4. Gene map – Signaling/regulatory pathways involved in the adipogenesis process
