
// Project
The DeLIMIT project
Duration of the project
18 months
Financing contract
100 PED/03.01.2017
Coordinator
Emergency County Clinical Hospital "Pius Brînzeu" Timișoara - OncoGen
Partner
University of Medicine and Pharmacy "Victor Babeș" Timisoara
Project details
The specific objectives pursued in the first stage of implementation of the DeLIMIT project
Generation of breast and colon cancer tumor spheroids, fabrication of multicellular spheroids by 3D bioprinting and the in vitro characterization of tumor models.
The specific objective of the second stage of the project
Validation in vivo of the optimal tumor model made by 3D bioprinting, which included the in vivo evaluation of the evolution of tumor models obtained by 3D bioprinting and computational modeling / simulation of cell rearrangement after bioprinting.
Project summary:
In this project, we aim to make three-dimensional (3D) tissue constructs that mimic the tumor microenvironment. These will include a multicellular system, whose structure will be similar to the tumor, respectively a shell of cells embedded in the extracellular matrix, which represents the vicinity of the tumor.
To assemble them, we will develop 3D tissue bioprinting methods, a method that belongs to tissue engineering. We will build 3D models of tumors in their environment and validate them, characterizing them from a morphological, phenotypic, genotypic and functional point of view. We propose to use two cell sources: standard cell lines as well as primary cells. The first option is more reproducible, and the second one is more likely to provide more realistic tumor models. For each cell type, we will test two computer-assisted assembly methods: the bioprinting of hydrogels with cellular content in suspension, and the bioprinting of cellular aggregates. The first method ensures a better resolution, and the second leads to a higher cell density.
The obtained tumor models will be validated on immunosuppressed mice. Prior to bioprinting, malignant cells and peritumoral fibroblasts will be stably transfected with GFP, so that tumors can be visualized by fluorescent microscopy in vivo. Also, the tissue constructs will be ranked according to their ability to mimic the secretory behavior of tumors in vivo.
Following validation, the project results will be applicable in studies of cancer therapies: the 3D tumor models will be usable for personalized testing of the effectiveness of some pharmacological agents, in studies of tumor evolution, as well as in studies aiming at the transfer of in vitro and in vivo results in clinical trials, in the broad context of antitumor therapies.
Results:
We validated the 3D bioprinting methodology of tumor models in vitro, using 3 distinct cell types, from the peri-tumoral environment (TAF and T lymphocytes) and tumor cells; 2 distinct tumor models were validated in vitro: the triple stratified model and the torsional model; We validated in vivo the triple-stratified 3D bioprinted tumor model on immunosuppressed CD1 Nu/Nu mice; validation of tumor development was done in vivo with the help of the Hamamatsu Aequoria System imaging system (quantification of tumor development), and after tumor explantation, the in vitro model was validated, by highlighting the cellular distribution and the expression of characteristic markers; The computational model for tumor development simulation was validated.
-
- Experimental procedure for making tumor spheroids in vitro (Fig. 1)
- Bioprinting method of toroidal (Fig. 2A) and triple-layered (Fig. 2B-D) tumor models
- In vitro cultivation process of bioprinted tissue models (Fig. 4)
- Procedure for in vivo cultivation of tissue models by subcutaneous implantation in immunosuppressed mice (CD1 Nu/Nu) (Fig. 5)
- G-code programs for bioprinting the tissue models represented in Fig. 2
- Computational models of cellular resolution of tissue structures and programs for simulating their evolution (Fig. 3)

Fig. 1. HT-29 colon cancer tumor spheroids visualized by stereomicroscopy (marker = 200 micrometers)
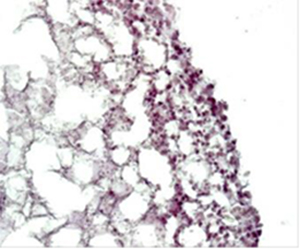
Fig. 4. HE staining of bioprinted tumor models after 6 days of in vitro cultivation
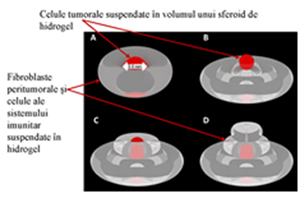
Fig. 2. Digital models of the developed tissue structures: toroidal model (A) and triple-layered model (BD).
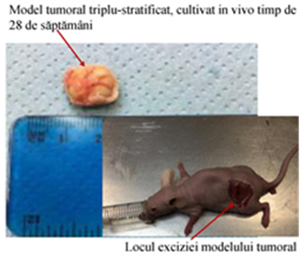
Fig. 5. Triple-layered tumor model cultured in vivo
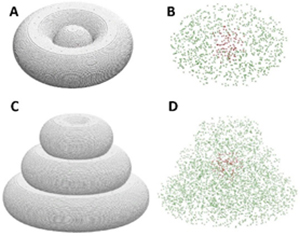
Fig. 3. Computational models of the tissue structures made on a 1:1 scale, visualized with (A, C) and without the dispersing hydrogel (B, D).
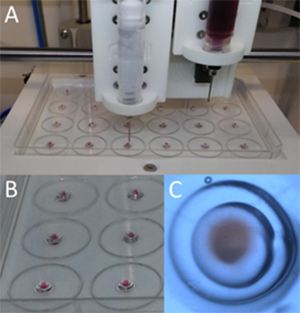
Fig. 6. Experiment to optimize the printing process made with two types of hydrogel
Participation in scientific events:
- Neagu A, Bojin F, Bejenariu MI, Neagu M, Cristea A, Popescu R, Păunescu V. 3D bioprinting techniques for building model tissues" - oral lecture, 29th National Conference of the Romanian Physiological Society, 25 - 27 May 2017 , Timisoara.
- Bojin F, Neagu A, Bejenariu MI, Cean A, Popescu R, Neagu M, Cristea A, Păunescu V. "3D bioprinting techniques for building model tissues that mimic the tumor microenvironment" - oral lecture, BIOFABRICATION 2017 International Conference on Biofabrication, 15 - October 18, 2017, Beijing, China. more important achievements from the first stage of project implementation.
- Bojin F, Bejenariu MI, Robu A, Cean A, Popescu R, Neagu M, Gavriliuc O, Neagu A, Păunescu V. "Bioprinted models of the tumor microenvironment: in vivo evaluation and computer simulations" - oral lecture, BIOFABRICATION 2018 International Conference on Biofabrication, 28-31 October 2018, Würzburg, Germany.
- Neagu A, Brakke K, Robu A, Bejenariu MI, Koudan E, Bulanova E, Parfenov V, Hesuani Y, Kharin A, Timoshenko V, Mironov V, "Sacrificial multicellular spheroids (sacrospheres) for the biofabrication of tubular tissue constructs", poster, BIOFABRICATION 2017 International Conference on Biofabrication, October 15-18, 2017, Beijing, China.
Full articles:
- Neagu A. (2017) Role of computer simulation to predict the outcome of 3D bioprinting. Journal of 3D Printing in Medicine 1(2):103-121.
- Robu A, Robu N, Neagu A. (2018) New software tools for hydrogel-based bioprinting. Proceedings of SACI 2018, IEEE 12th International Symposium on Applied Computational Intelligence and Informatics, May 17-19, 2018, Timisoara, Romania. – full article published in the Proceedings volume of the conference (DOI: 10.1109/SACI.2018.8440971).
